Key Highlights about USDeN:
- Pilot and Grant Planning Awards (Letter of Intent (LOI) due January 4, 2023)
- Junior Investigator Intensive Program (Application due February 1, 2023)
- USDeN Annual Meeting (May 3, 2023—Long Beach, CA)
- USDeN Webinars (Future schedule & Links to previously recorded)
Cynthia M. Boyd, MD, MPH, the Mason F. Lord Professor of Medicine, is a Senior Associate with the Center on Aging and Health. Dr. Boyd’s primary appointment is with the School of Medicine, where she serves as the Director of the Division of Geriatric Medicine and Gerontology, and she has joint appointments with the Bloomberg School of Public Health in Health Policy and Management and in Epidemiology. Dr. Boyd’s research interests at Johns Hopkins include older people, person- and family-centered care multiple chronic conditions, multimorbidity, disability, functional recovery, quality of life, cognitive decline, polypharmacy, deprescribing, and guidelines. Also, Dr. Boyd is co-primary investigator for the US Deprescribing Research Network (USDeN) along with Dr. Michael Steinman with the University of California, San Francisco. Today, we will speak with her about USDeN.
Tony Teano: Thanks for taking the time for this interview. You are a very well-respected national leader in the field of Geriatric Medicine and you are internationally known for your leadership in deprescribing research among older adults. What is deprescribing and why is it important to older adults?
Dr. Boyd: My pleasure! I’m always happy to talk about the importance of deprescribing to older adults! Deprescribing refers to the thoughtful and systematic process of identifying high risk, low benefit, or unnecessary medications and either reducing the dose or stopping these medications in a manner that is safe, effective, and helps people maximize their wellness and goals of care. Deprescribing has the potential to avoid harms and to increase the quality of life.
Deprescribing is a collaborative effort between a patient and a health care professional to optimize medication usage in a way that puts what matters most to the patient as the goal, in tandem with reducing or eliminating potentially harmful medications. Medications may interact with each other in a way where the risks of harm outweigh the benefits. Some medications may no longer be needed because the condition may have resolved. Some medications may metabolize differently in an older adult compared to the way their body processed them when they were originally prescribed, and that may yield undesirable side-effects—so a medication may need to be decreased appropriately. Some medications, such as those prescribed in an emergency room or at hospital discharge, may have only been meant to be temporary. Plus, one medication may lead to a side-effect that causes a person to take yet another medication. This is especially important for older adults to consider, particularly if they are taking multiple prescriptions for multiple chronic conditions that may have been prescribed by multiple physicians over time.
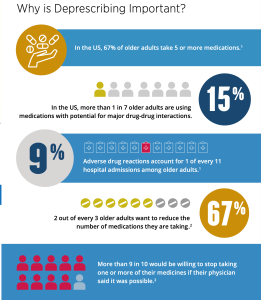
Tony Teano: What are a few common examples of medications that some older adults might be better off without? Perhaps some medications that are red flags to check?
Dr. Boyd: Some medications, such as certain antihistamines and anti-anxiety drugs, may increase the risk of falls among older adults, and it may be more important for that individual to stop taking or cut back on such medications. Another example is that some medications may have side-effects that increase the risk of confusion or even delirium among older adults, such as opioids and some prescription sleep aids. Other commonly overly prescribed medications include proton pump inhibitors and gabapentin.
There are many drugs that may no longer be of benefit to a patient, and lists of medications should be reviewed for potential interactions and side-effects and whether they are still needed. It is crucially important to mention that exploring tapering off or eliminating such medications should only be done under the close supervision of a doctor or other prescriber such as a nurse practitioner, physician assistant. Pharmacists are a great help in reviewing combinations of medications. Together with a patient, health care professionals should assess each person’s individual care plan and focus on what matters most to that person, and to deprescribe, with follow up and monitoring when appropriate.
Reviewing medications to evaluate for the possibility of deprescribing should be a routine practice to support safety and personalized care: improve quality of life for patients, optimize the use of medications that are necessary, and putting what matters most to the patient and their health outcomes at the center of the conversation. For these reasons, deprescribing is not only a science backed by evidence and research, it is also an art in the sense that no two pictures of it may look quite the same because each person is unique and values different treatment priorities. Deprescribing is tailored care.
Reviewing medications periodically to screen for drug interactions and patient-centered care priorities should be a normal part of care but it doesn’t always actually happen. These conversations to are essential to high-quality care and in the best interest of older adults. Clinicians need more research, guidelines, and best practices about how to do this well. And that’s what the US Deprescribing Research Network (USDeN) is here for—providing rigorous, evidence-based research to support clinicians, patients, and their caregivers—all of whom are essential stakeholders in any deprescribing conversation.
Tony Teano: Please tell us more about USDeN and what it aims to do.
Dr. Boyd: Funded by the US National Institute on Aging, USDeN’s goal is to develop and disseminate evidence about deprescribing for older adults, and in doing so to help improve medication use among older adults and the outcomes that matter. USDeN is a community of people interested in mutual collaboration and learning about improving the body of impactful research on deprescribing for older adults. Overall, our activities are aimed at providing meaningful, helpful resources to catalyze expansion of the quality, quantity, and ultimate impact of deprescribing research. To this purpose, USDeN has four robust, highly-engaged cores: Investigator Development Core; Pilot and Exploratory Studies Core; Stakeholder Engagement Core; and the Data and Resources Core.
I encourage anyone interested in USDeN to join us at our next annual meeting, which will be conveniently held just ahead of the American Geriatrics Society’s annual meeting, on Wednesday May 3rd in Long Beach, California (more information here). This meeting will comprise a wide-ranging series of sessions and activities that are focused on:
- Enhancing skills, providing multidisciplinary perspectives, and offering practical guidance on and opportunities for deprescribing research;
- Communicating how the network can help an investigator advance deprescribing research interests;
- Building collaborations and community among people interested in deprescribing research—and how it can be used to improve care for older adults.
 The annual network meeting is open to all who are interested in research on deprescribing for older adults, including early-stage investigators, more experienced investigators, patients, caregivers, health system and other stakeholders, and policymakers who have a strong interest in advancing scholarship on deprescribing and translating research findings into everyday practice. The meeting is designed to be interactive, so all attendees should be prepared to actively participate!
The annual network meeting is open to all who are interested in research on deprescribing for older adults, including early-stage investigators, more experienced investigators, patients, caregivers, health system and other stakeholders, and policymakers who have a strong interest in advancing scholarship on deprescribing and translating research findings into everyday practice. The meeting is designed to be interactive, so all attendees should be prepared to actively participate!
Tony Teano: I noticed that USDeN offers grant awards. Tell us about them, please.
Dr. Boyd: Yes! USDeN has Pilot and Grant Planning award opportunities, and the Letter of Intent for this cycle is on January 4, 2023. The LOI is really just a quick way to let USDeN know that you are interested and help us plan for our scientific review process—it doesn’t have to be a detailed plan. The LOI is required, and the January 4, 2023 deadline is firm. So I encourage anyone interested in this field to get it done soon! You can find examples of all the required documents and a webinar tutorial about it on USDeN’s website. The LOI is an easy first step to do.
All topics related to deprescribing research are welcome. Pilot awards are helpful to goals such as gathering preliminary data, proof of concept, or development work for a future, large-scale study. Pilot awards also support career development, especially for junior investigators. Pilot awards are for 1 year with a maximum budget of $60,000 in total costs. Grant Planning awards are intended to fund grant preparation activities that will lead to submission of large grant proposals, such as multisite clinical trials. For instance, a Grant Planning award might support funding for meetings and travel for study investigators to refine an intervention for different sites, and/or research costs to engage multiple clinical sites in a multisite research trial. We are interested in stakeholder engagement being a robust force behind all the research we do. You can find more information and resources on how to incorporate stakeholder engagement in the planning and conduct of your research here.
Tony Teano: This grant seems like a great opportunity, especially for those breaking into the field of deprescribing research. What additional ways does USDeN have to help investigators as they begin to enter this specialty?
Dr. Boyd: We love developing future deprescribing research investigators! One of the USDeN programs of which I am most proud is the Junior Investigator Intensive (JII) Program in Deprescribing Research, and the 2023-2024 application cycle is open! The applicant due date is February 1, 2023. (For details, click here.) The JII program will cultivate a cohort of emerging deprescribing research leaders. The JII program has three main components:
- Scholars will attend a special additional workshop at the 2023 US Deprescribing Research Network Annual Meeting that is focused on career development, networking, and collaborative research opportunities for early-stage investigators interested in deprescribing.
- Scholars will attend monthly “work-in-progress” meetings, a core curriculum, and other activities over the year that offer a mix of opportunities to get feedback on your research from colleagues and senior researchers and discussion of collaborative research projects in which scholars can participate (and help lead).
- Scholars will have access to other aspects of USDeN, such as attendance at webinars, consultations, and engage with a robust community of other junior investigators in deprescribing research from around the world. Scholars will be expected to attend the USDeN Annual Meeting and most of the monthly web-based meetings during the year.
Additionally, we offer webinars with fabulous international leaders in deprescribing. All of that is freely, publicly available to anyone interested in deprescribing research, and the recordings of the webinars are available from our website. Our next webinar is a prime example of a hot topic in deprescribing research with a leading expert; it will be on January 17th and it is about the “Complex challenges in pain and opioid management: Relevance to deprescribing,” and presenting will be Dr. Jessica S. Merlin, MD, PhD, MBA. Dr. Merlin is an Associate Professor of Medicine at the University of Pittsburgh School of Medicine, Director of CHAMPP (CHAllenges in Managing and Preventing Pain) Research Center, and Co-Director of TREETOP (Tailored Retention and Engagement in Equitable Treatment of Opioid use disorder and Pain) Research Center. (Register here.)
Tony Teano: The more you talk about deprescribing, the more I wonder why there’s any hesitation to it. It just seems to make sense! What are some of the obstacles to deprescribing? Why is there any hesitancy?
Dr. Boyd: This is a very important question, and we need more research around it. Essentially… Awareness. Trust. Communication. Teamwork. These are among the top key challenges to the art of deprescribing. Many people may not understand that as they age, their metabolism changes and their bodies no longer process the drugs the way they used to—and the chemicals stay in their systems longer than before. Moreover, as people age and face new diagnoses over time, an increasing number of medications tend to be prescribed—and some of those may have negative interactions with each other, or cause serious side-effects. Consequently, the patient may not know where to begin to make their medicine regime more simple and safe, or to understand any potential for harm—especially if they have several doctors acting in silos and prescribing medications without getting the bigger picture…. So, education is very important as a starting point—for clinicians as well as patients and their caregivers who may assist them with managing medications. Trust and communication go hand in hand as a foundation for sharing information and making smart decisions about an approach to deprescribing. Sometimes, there’s a cultural disconnect between a patient and a provider. Sometimes, a doctor may not have a history with a new patient and must establish a collaborative approach to care. And deprescribing is a team effort that includes a lot of people in the support system of an individual’s care who also need to be aware of drug interactions—from the pharmacist filling prescriptions to the emergency department doctors who see a patient in an urgent situation, and nurses who may administer medications during a hospital admission, and so forth.
Tony Teano: Dr. Boyd, I appreciate your taking the time to give us a “Deprescribing 101” crash course! This is a lot of great information to highlight why it is important for older adults to regularly review their medications with their doctor for optimal care and safety. Is there anything else you’d like to share with our readers?
Dr. Boyd: If you’d like more information about deprescribing research, please visit USDeN’s website or email us. We’re here to facilitate advancing research to optimize medication use among older adults. Let us know how we can support you in this cause.
By Anthony L. Teano, MLA
Communication Specialist

